SAS Voices
News and views from the people who make SAS a great place to work
Collecting, managing, standardizing and analyzing clinical data during (and after) a clinical trial is crucial in the process for submission and regulatory approval of a new compound, biological, device or other therapy. A central clinical platform requires: Robust and auditable analytics to prove the result to the authorities and external

“Good afternoon, Mr. Yakamoto. How did you like that three-pack of tank tops you bought last time you were in?” Washington D.C. Year 2054. Chief of PreCrime John Anderton is running from the law for a crime he has not committed yet. After a risky eye transplant in order to
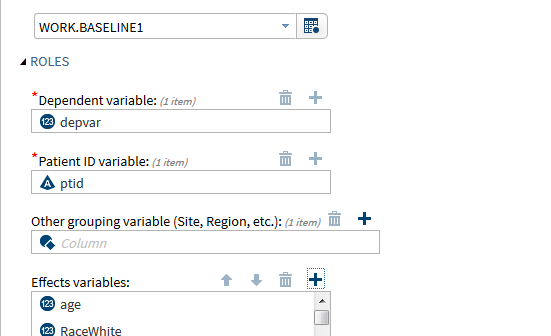
SAS is widely used in clinical research activities including: Managing and transforming data. Generating tabular and graphical summaries. Performing powerful statistical analyses such as safety and efficacy evaluations. In addition, SAS provides a number of interfaces from which a user can select to work with the data. One of these


