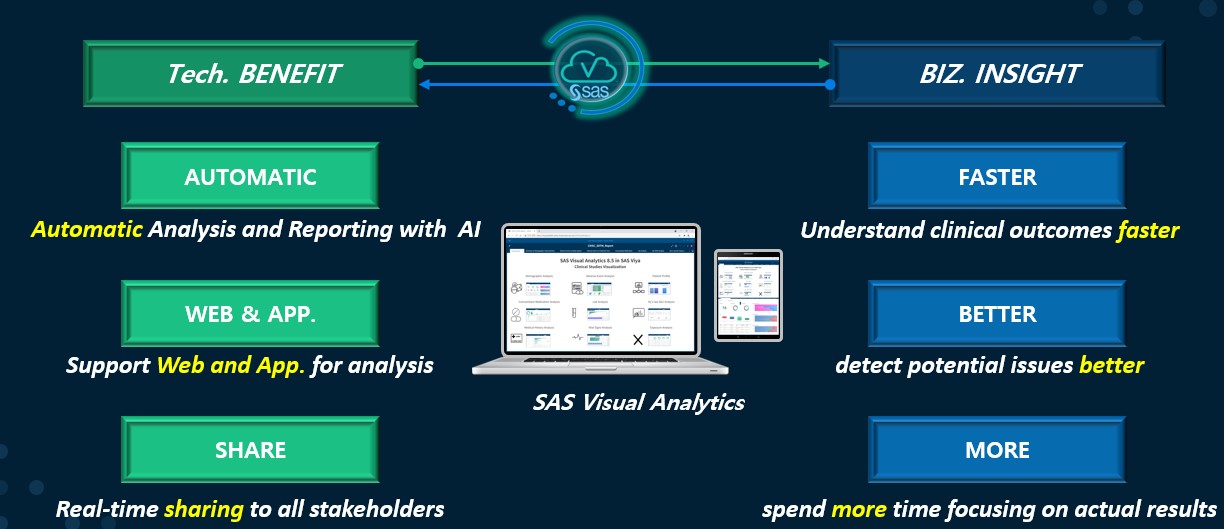
지난 블로그 포스팅 #1편에서는 임상시험 전 과정에 참여한 내.외부 모든 이해관계자가 임상시험 데이터에 쉽게 접근하여 진행 상황을 파악할 수 있도록 지원하는 SAS Visual Analytics 솔루션의 기능을 소개해 드렸습니다. 이번 포스팅에서는 이러한 AI기반의 SAS Visual Analytics 분석 솔루션을 활용하여 임상시험 SDTM 데이터의 탐색 및 시각화 리포트의 활용에 대해 알아보겠습니다. Clinical Data

지난 블로그 포스팅 #1편에서는 임상시험 전 과정에 참여한 내.외부 모든 이해관계자가 임상시험 데이터에 쉽게 접근하여 진행 상황을 파악할 수 있도록 지원하는 SAS Visual Analytics 솔루션의 기능을 소개해 드렸습니다. 이번 포스팅에서는 이러한 AI기반의 SAS Visual Analytics 분석 솔루션을 활용하여 임상시험 SDTM 데이터의 탐색 및 시각화 리포트의 활용에 대해 알아보겠습니다. Clinical Data

When Jack Shostak and I first started thinking about writing a SAS book on implementing CDISC (Clinical Data Interchange Standards Consortium) standards, we held one truth to be self-evident: that at least some parts of the book would be outdated before it was even published. Thanks to some lucky timing
There’s always plenty to learn at PharmaSUG, which is one reason it draws attendees not only from the US and Canada, but from countries such as Denmark, Finland, Germany, India, Israel, Italy, Japan, Mexico and the UK. Learning Highlights Technical Keynote Address: "CDISC Standards: Now and To Come" from Wayne