Collecting, managing, standardizing and analyzing clinical data during (and after) a clinical trial is crucial in the process for submission and regulatory approval of a new compound, biological, device or other therapy. A central clinical platform requires:
- Robust and auditable analytics to prove the result to the authorities and external stake holders such as patient communities and physicians.
- A controlled environment (Figure 1) that fosters collaboration between the different groups in clinical development such as data management, biostatisticians, clinical writers and scientific investigators.
- Enhancing your platform with new functions and integration capabilities to meet the needs of a clinical development group.
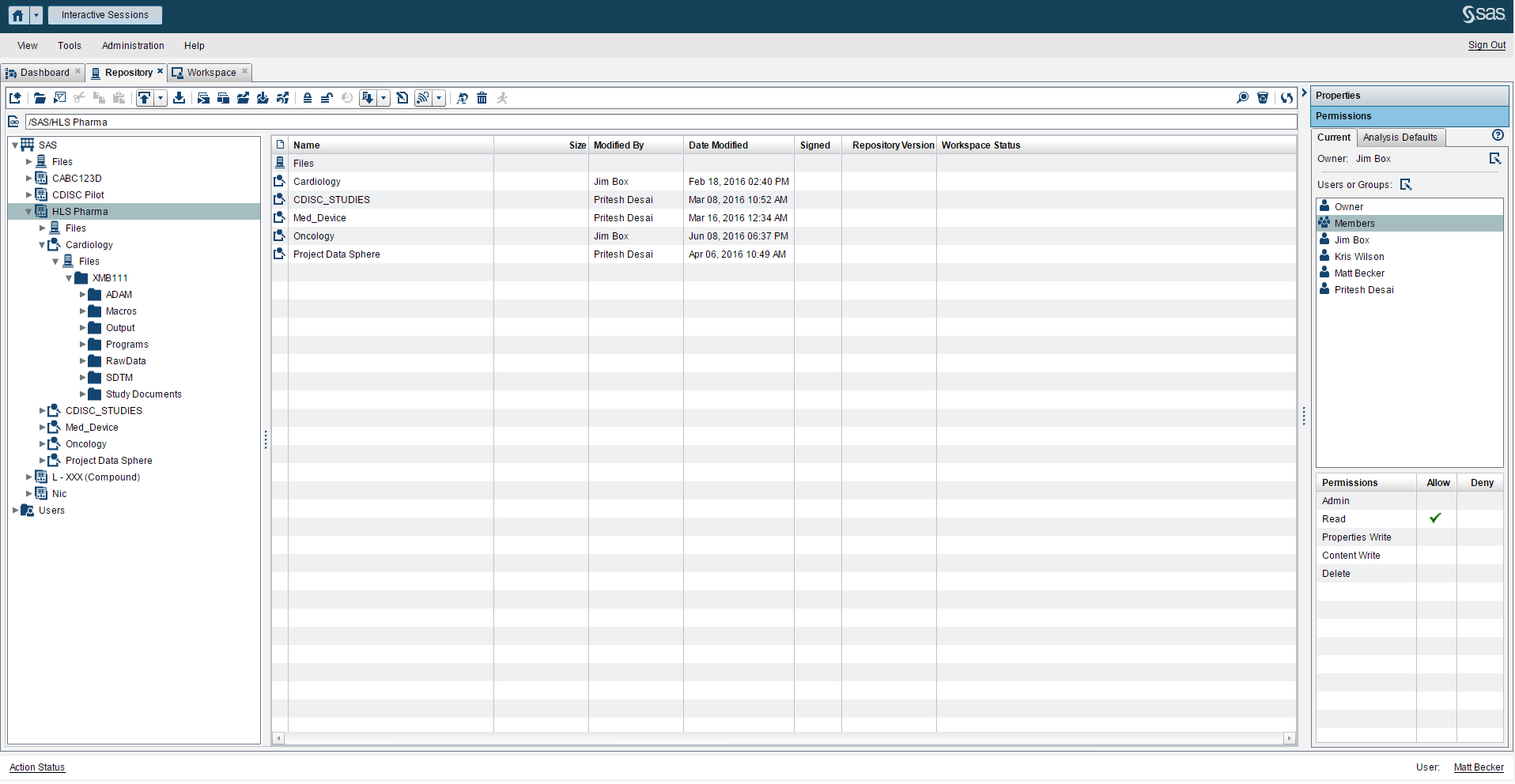
Beyond providing a reliable way to access data, programs and clinical data standards (Figure 2), you need a way to satisfy these three requirements. SAS® Life Science Analytics Framework is a modern services-based extensible platform that allows organizations to integrate and extend capabilities for CDISC metadata management and facilitates integration with another system such as an EDC system.
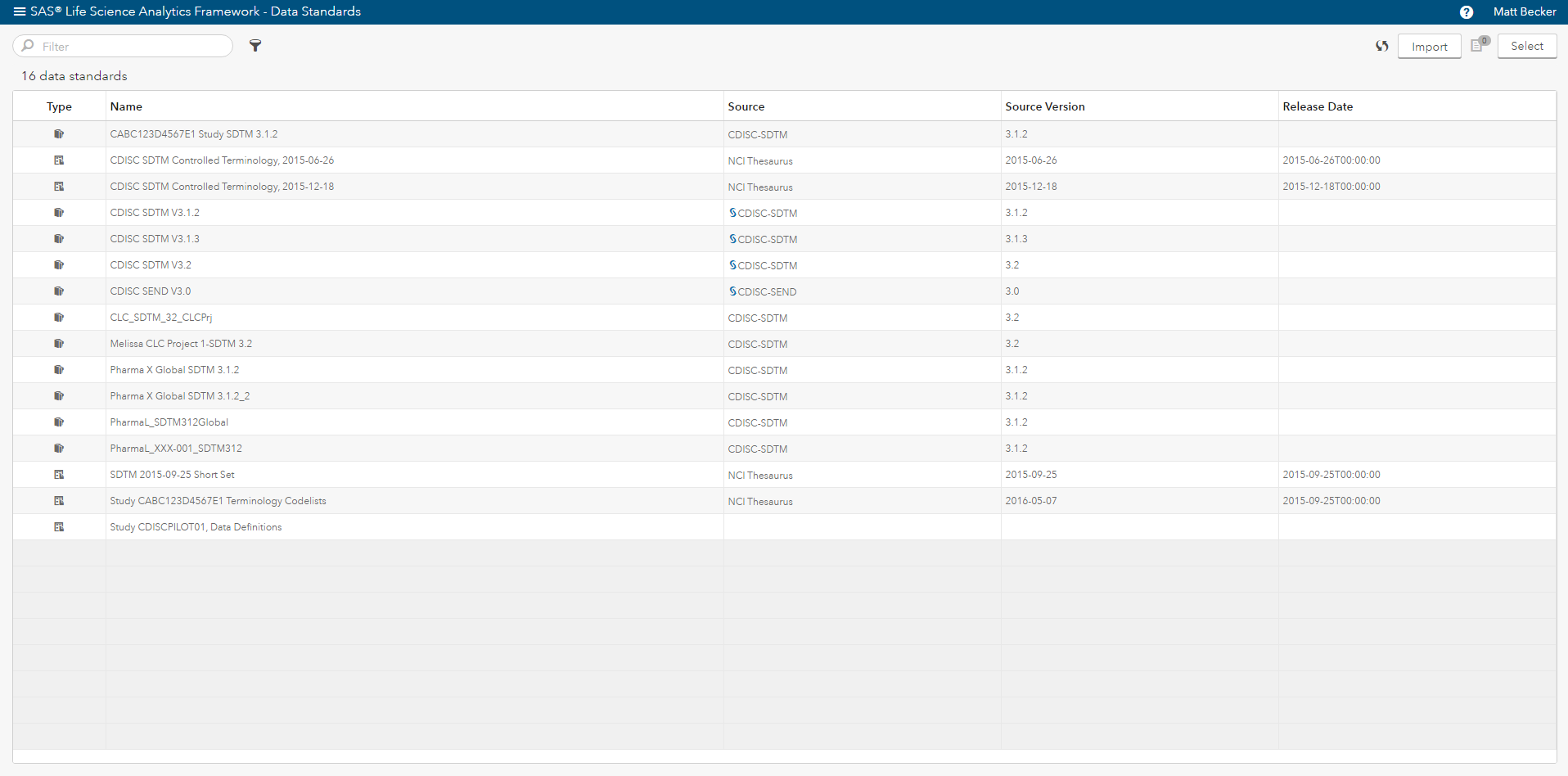
In addition, SAS provides the solutions and the underlying technology to:
- Standardize collected clinical data.
- Easily combine and transform it into readily analyzable data sets.
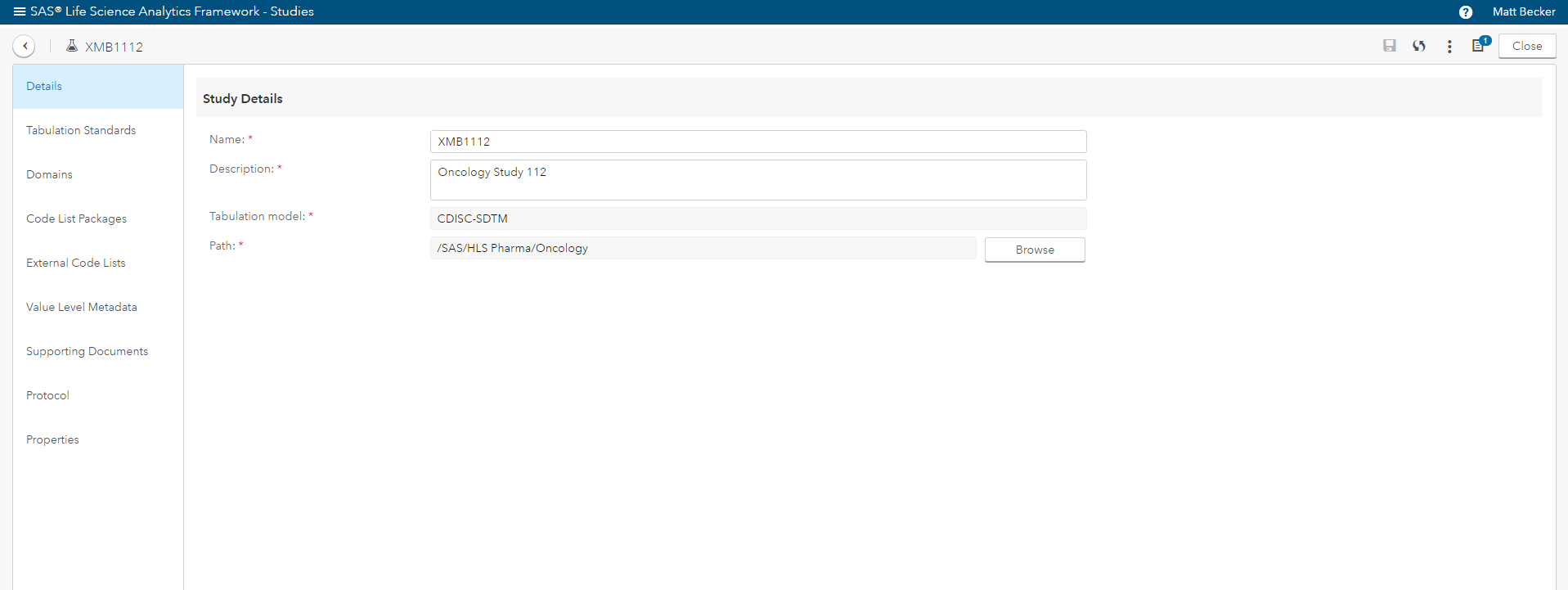
- Manage (in detail) the CDISC standards in the context of clinical studies (Figure 3).
- Enable statisticians to analyze the data with the highest scientific rigor.
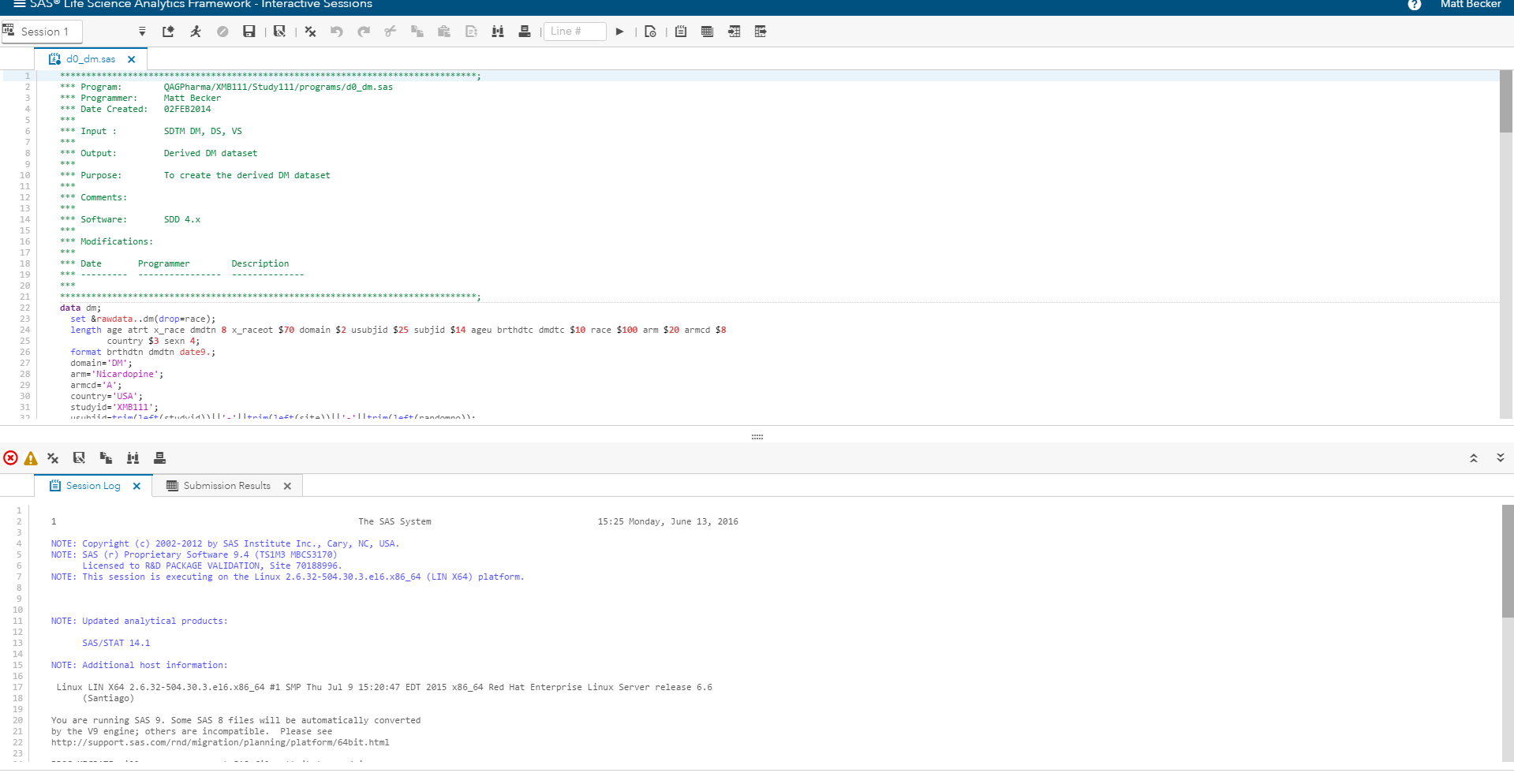
SAS provides the analytics to optimize the processes and resources required to move a new molecule or therapy from the laboratory to the clinic. SAS also hosts the integrated analytics environment (Figure 4) in a secure, private cloud and handles all management and maintenance of the complex IT requirements for a strongly audited and regulated system.
Want to learn more? At the upcoming DIA 2016 Annual Meeting in Philadelphia, we will have experts available to demonstrate the full capabilities of LSAF. Please stop by Booth 1825 to discuss how you may be able to use SAS Life Science Analytics Framework in your organization.
