There was this very embarrassing day around year six of my career as a statistician working in clinical trials. I had a small group of interns working on a project that combined data from multiple clinical trials. The goal was to better understand sources of variation in the common control
Tag: dia
There was this very embarrassing day around year six of my career as a statistician working in clinical trials. I had a small group of interns working on a project that combined data from multiple clinical trials. The goal was to better understand sources of variation in the common control
Collecting, managing, standardizing and analyzing clinical data during (and after) a clinical trial is crucial in the process for submission and regulatory approval of a new compound, biological, device or other therapy. A central clinical platform requires: Robust and auditable analytics to prove the result to the authorities and external
Collecting, managing, standardizing and analyzing clinical data during (and after) a clinical trial is crucial in the process for submission and regulatory approval of a new compound, biological, device or other therapy. A central clinical platform requires: Robust and auditable analytics to prove the result to the authorities and external

SAS is widely used in clinical research activities including: Managing and transforming data. Generating tabular and graphical summaries. Performing powerful statistical analyses such as safety and efficacy evaluations. In addition, SAS provides a number of interfaces from which a user can select to work with the data. One of these
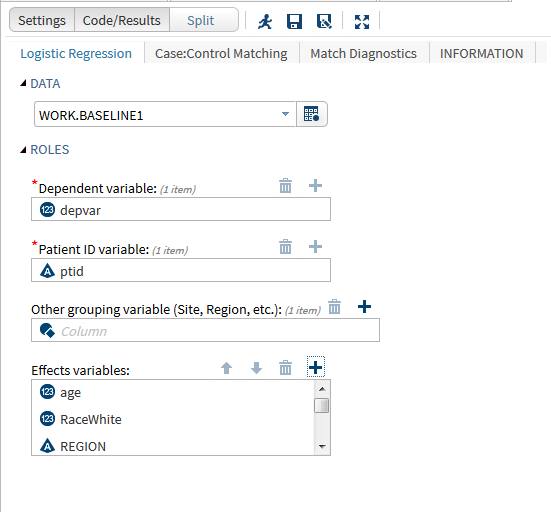
SAS is widely used in clinical research activities including: Managing and transforming data. Generating tabular and graphical summaries. Performing powerful statistical analyses such as safety and efficacy evaluations. In addition, SAS provides a number of interfaces from which a user can select to work with the data. One of these


