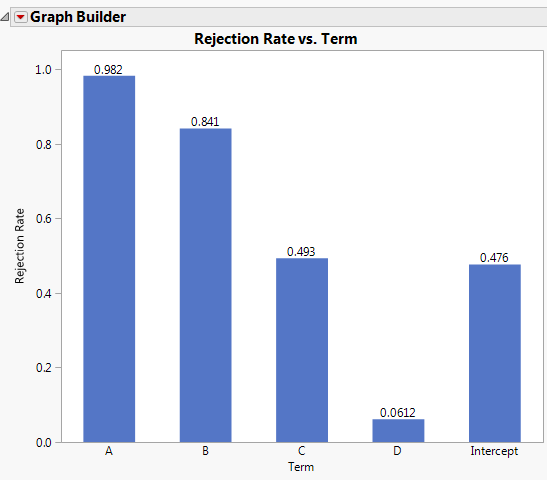
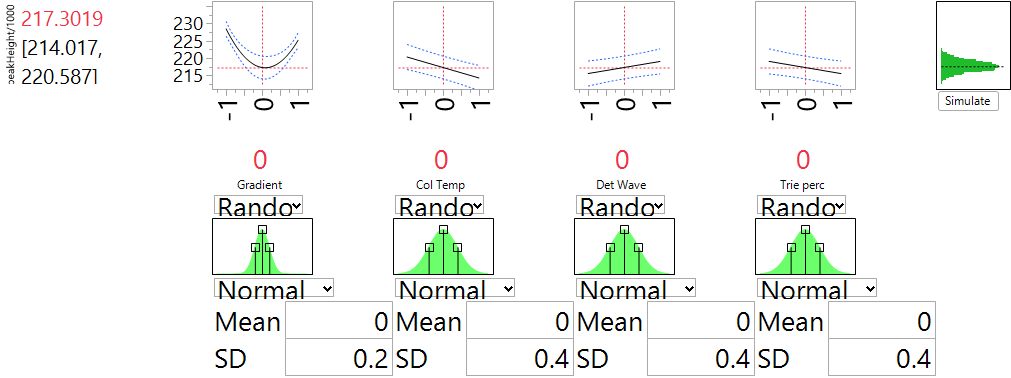
Ronald Snee and Roger Hoerl have written a book called Strategies for Formulations Development. It is intended to help scientists and engineers be successful in creating formulations quickly and efficiently. The following tip is from this new book, which focuses on providing the essential information needed to successfully conduct formulation studies in the